Adrenocortical Carcinoma Market Forecast upto 2034—Insights into Emerging Therapies and Market Trends | DelveInsight
The adrenocortical carcinoma market in the 7MM is anticipated to boost during the forecast period (2025–2034), due to the launch of emerging therapies such as CY 101 from Cytovation and OR 449 from Orphagen Pharmaceuticals, and others, and the increasing cases of ACC.
New York, USA, July 29, 2025 (GLOBE NEWSWIRE) -- Adrenocortical Carcinoma Market Forecast upto 2034—Insights into Emerging Therapies and Market Trends | DelveInsight
The adrenocortical carcinoma market in the 7MM is anticipated to boost during the forecast period (2025–2034), due to the launch of emerging therapies such as CY‑101 from Cytovation and OR‑449 from Orphagen Pharmaceuticals, and others, and the increasing cases of ACC.
DelveInsight’s Adrenocortical Carcinoma Market Insights report includes a comprehensive understanding of current treatment practices, emerging adrenocortical carcinoma drugs, market share of individual therapies, and current and forecasted adrenocortical carcinoma market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Adrenocortical Carcinoma Market Report
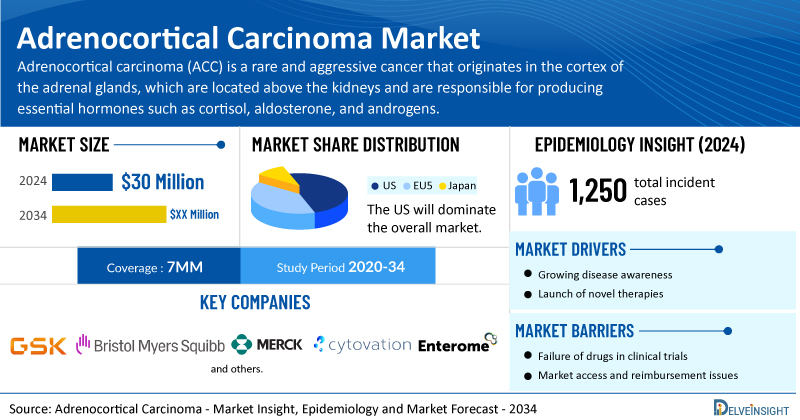
- According to DelveInsight’s analysis, the total adrenocortical carcinoma market size is expected to grow from USD 30 million in the 7MM in 2024.
- The United States accounts for the largest market size of adrenocortical carcinoma, in comparison to EU4 (Germany, Italy, France, and Spain) and the UK, and Japan.
- Based on DelveInsight's assessment in 2024, the 7MM had 1,250 incident cases of ACC, and it is projected that these cases will experience an increasing growth trend with a significant CAGR during the forecast period (2025−2034).
- Prominent companies, including GlaxoSmithKline, Bristol Myers Squibb, Merck, Cytovation, Enterome, Orphagen Pharmaceuticals, and others, are actively working on innovative adrenocortical carcinoma drugs.
- Some of the key adrenocortical carcinoma therapies in the pipeline include CY-101, OR-449, dostarlimab, nivolumab + ipilimumab, and pembrolizumab, EO2401 + Nivolumab, and others. These novel adrenocortical carcinoma therapies are anticipated to enter the adrenocortical carcinoma market in the forecast period and are expected to change the market.
Discover which adrenocortical carcinoma medications are expected to grab the market share @ Adrenocortical Carcinoma Market Report
Adrenocortical Carcinoma Market Dynamics
The adrenocortical carcinoma market dynamics are anticipated to change in the coming years. The availability of surgical options such as adrenalectomy provides a practical approach for removing the diseased adrenal gland, thereby improving access to treatment. Mitotane remains the mainstay treatment for ACC, with its exclusive status as an 'Off-Patent, Off-Exclusivity Drug without an Approved Generic' in the Orange Book reinforcing its dominant position.
There is significant potential for collaboration among medical professionals, researchers, and patient advocacy organizations to raise disease awareness and broaden access to care. The limited therapeutic landscape for ACC presents a strategic opportunity for new players to enter and expand within the market.
Furthermore, many potential therapies are being investigated for the treatment of adrenocortical carcinoma, and it is safe to predict that the treatment space will significantly impact the adrenocortical carcinoma market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the adrenocortical carcinoma market in the 7MM.
However, several factors may impede the growth of the adrenocortical carcinoma market. The reliance on surgery as the main treatment for ACC, due to the limited availability of systemic therapies, highlights a weakness stemming from a lack of diverse treatment options. ACC's rarity and the small number of affected patients make it difficult to conduct large-scale clinical trials, complicating the development of evidence-based treatment protocols and assessment of new therapies.
Resistance to current treatments like mitotane monotherapy, or their limited effectiveness, often leads to poor patient outcomes and represents a major challenge. Additionally, the ultra-rare nature of ACC limits access to funding and resources for research and development, posing a significant barrier to progress.
Moreover, adrenocortical carcinoma treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the adrenocortical carcinoma market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the adrenocortical carcinoma market growth.
Adrenocortical Carcinoma Treatment Market
Surgical removal is the primary treatment option for localized adrenocortical carcinoma. However, many patients are diagnosed at advanced stages, where surgery alone is unlikely to be curative. Chemotherapy, particularly mitotane, is the only FDA-approved drug for ACC, though its effectiveness varies, and it often causes substantial side effects.
Complete surgical excision remains the only potentially curative option. For localized cases, adjuvant therapies are used to reduce the risk of recurrence. In cases where the tumor is unresectable or has metastasized, treatment shifts to palliative care.
First-line treatment strategies for ACC are personalized, taking into account the patient's prognostic profile. For those with good performance status and aggressive disease, a combination regimen like EDP-M (etoposide, doxorubicin, cisplatin, and mitotane) is often employed. Patients with less aggressive disease may be treated with mitotane alone, sometimes alongside locoregional interventions.
Despite some efficacy, EDP-M is not curative, and the overall prognosis remains poor. Second-line options such as streptozocin with mitotane (S+M) and gemcitabine with capecitabine (G+C) show limited effectiveness, with response rates around 10%. G+C is generally well tolerated but lacks strong molecular predictors of response.
Temozolomide, used as a third-line option, shows anti-cancer activity in laboratory settings. While some patients experience disease stabilization, its clinical benefit is modest, and research is ongoing to determine which patient subgroups may respond best.
Looking ahead, future treatment advances may include new chemotherapies, angiogenesis inhibitors, and targeted small-molecule drugs. These innovations, driven by deeper molecular insights into ACC biology, offer potential for improved therapeutic outcomes.
Learn more about the adrenocortical carcinoma treatment options @ Adrenocortical Carcinoma Drugs Market
Adrenocortical Carcinoma Emerging Drugs and Companies
Research and development efforts in adrenocortical carcinoma remain limited due to its classification as an ultra-rare disease, resulting in a sparse pipeline with only a few therapies currently in Phase II, Phase I/II, or preclinical stages. Among the investigational treatments being evaluated in clinical trials are Dostarlimab (GlaxoSmithKline), the combination of Nivolumab and Ipilimumab (Bristol Myers Squibb), Pembrolizumab (Merck), CY-101 (Cytovation), EO2401 combined with Nivolumab (Enterome/Bristol Myers Squibb), OR-449 (Orphagen Pharmaceuticals), and others.
CY-101 is designed to selectively attack and destabilize cancer cell membranes, which leads to the release of tumor-specific neoantigens and enables the immune system to launch a targeted, systemic response against the cancer. In addition, CY-101 blocks the β-catenin signaling pathway, thereby impeding tumor progression and further activating immune defenses. Currently being developed by Cytovation, CY-101 is slated to enter a multi-national Phase II clinical trial for adrenocortical carcinoma in late 2025, with initial data expected in 2026. This study is being conducted in collaboration with Cancer Research UK and the Norwegian Cancer Society, as announced in January 2025.
OR-449 is a first-in-class, orally available inhibitor of the orphan nuclear receptor steroidogenic factor-1 (SF-1, NR5A1), a validated target in ACC—a rare and difficult-to-treat adrenal gland cancer. The U.S. FDA has granted OR-449 Rare Pediatric Disease Designation (RPDD) to support its expedited development for pediatric ACC. Additionally, Orphagen has secured a $10.2 million grant from the Cancer Prevention and Research Institute of Texas (CPRIT) to fund its IND application and a Phase I trial in adults with ACC. The grant spans three years and requires Orphagen to raise matching funds.
Pembrolizumab, a widely approved PD-1 checkpoint inhibitor, is under evaluation in a Phase II trial for patients with advanced ACC. The trial, sponsored by Memorial Sloan Kettering Cancer Center, is currently in the "active, not recruiting" phase.
EO2401, a novel microbiome-based therapeutic vaccine developed by Enterome, was aimed at treating solid tumors such as glioblastoma and ACC. However, in November 2024, its Phase I/II SPENCER trial was terminated due to strategic considerations. This development marks a setback in the search for innovative treatments targeting ACC.
The anticipated launch of these emerging adrenocortical carcinoma therapies are poised to transform the adrenocortical carcinoma market landscape in the coming years. As these cutting-edge adrenocortical carcinoma therapies continue to mature and gain regulatory approval, they are expected to reshape the adrenocortical carcinoma market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for adrenocortical carcinoma, visit @ Adrenocortical Carcinoma Management
Recent Developments in the Adrenocortical Carcinoma Market
- In April 2025, Cytovation announced that it had raised NOK62 million (USD 6 million) largely from existing investors, led by Sandwater. These funds will be used to advance CY-101 into a multi-national Phase II clinical trial in patients with ACC.
- In November 2024, the status of the Phase I/II SPENCER study was updated to "terminated," with the reason cited as a strategic decision. This termination represents a setback in the emerging prospects for developing therapies for ACC.
Adrenocortical Carcinoma Overview
Adrenocortical carcinoma (ACC) is a rare and aggressive cancer that originates in the cortex of the adrenal glands, which are located above the kidneys and are responsible for producing essential hormones such as cortisol, aldosterone, and androgens. ACC can occur at any age but is most commonly diagnosed in children under 5 and adults in their 40s and 50s. The exact causes of ACC are not well understood, but it has been associated with certain genetic syndromes, including Li-Fraumeni syndrome, Beckwith-Wiedemann syndrome, and multiple endocrine neoplasia type 1 (MEN1), which suggests a hereditary component in some cases.
Symptoms of ACC can vary depending on whether the tumor is functioning (hormone-producing) or non-functioning. Functioning tumors may cause signs of hormone excess, such as Cushing's syndrome (weight gain, high blood pressure, muscle weakness, and skin changes), virilization in women (facial hair, deepened voice), or feminization in men. Non-functioning tumors may present later and often manifest as abdominal pain, a palpable mass, or symptoms related to tumor spread, such as weight loss or back pain.
Diagnosis of ACC typically involves a combination of imaging studies and laboratory tests. Imaging techniques such as CT scans or MRI help identify the size, location, and potential spread of the tumor. Hormonal blood and urine tests assess whether the tumor is producing excess hormones. A definitive diagnosis is usually confirmed through histological examination of tissue obtained via biopsy or after surgical removal of the tumor. Early detection is crucial, as ACC often presents at an advanced stage and has a poor prognosis if not treated promptly.
Adrenocortical Carcinoma Epidemiology Segmentation
The adrenocortical carcinoma epidemiology section provides insights into the historical and current adrenocortical carcinoma patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The adrenocortical carcinoma market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM, segmented into:
- Incident Cases of ACC
- Stage-specific Incident Cases of ACC
- Gender-specific Incident Cases of ACC
- Age-specific Incident Cases of ACC
Download the report to understand which factors are driving adrenocortical carcinoma epidemiology trends @ Adrenocortical Carcinoma Treatment Algorithm
| Adrenocortical Carcinoma Report Metrics | Details |
| Study Period | 2020–2034 |
| Adrenocortical Carcinoma Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Adrenocortical Carcinoma Market Size in 2024 | USD 30 Million |
| Key Adrenocortical Carcinoma Companies | GlaxoSmithKline, Bristol Myers Squibb, Merck, Cytovation, Enterome, Orphagen Pharmaceuticals, and others |
| Key Adrenocortical Carcinoma Therapies | CY-101, OR-449, dostarlimab, nivolumab + ipilimumab, and pembrolizumab, EO2401 + Nivolumab, and others |
Scope of the Adrenocortical Carcinoma Market Report
- Adrenocortical Carcinoma Therapeutic Assessment: Adrenocortical Carcinoma current marketed and emerging therapies
- Adrenocortical Carcinoma Market Dynamics: Conjoint Analysis of Emerging Adrenocortical Carcinoma Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Adrenocortical Carcinoma Market Access and Reimbursement
Discover more about adrenocortical carcinoma drugs in development @ Adrenocortical Carcinoma Clinical Trials
Table of Contents
| 1. | Adrenocortical Carcinoma Market Key Insights |
| 2. | Adrenocortical Carcinoma Market Report Introduction |
| 3. | Adrenocortical Carcinoma Market Overview at a Glance |
| 4. | Adrenocortical Carcinoma Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Adrenocortical Carcinoma Treatment and Management |
| 7. | Adrenocortical Carcinoma Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Adrenocortical Carcinoma Marketed Drugs |
| 10. | Adrenocortical Carcinoma Emerging Drugs |
| 11. | Seven Major Adrenocortical Carcinoma Market Analysis |
| 12. | Adrenocortical Carcinoma Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
Related Reports
Adrenocortical Carcinoma Pipeline
Adrenocortical Carcinoma Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key adrenocortical carcinoma companies, including Corcept Therapeutics, Bristol-Myers Squibb, Enterome, Exelixis, Ipsen, Genentech, among others.
Congenital Adrenal Hyperplasia Market
Congenital Adrenal Hyperplasia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key congenital adrenal hyperplasia companies, including Crinetics Pharmaceuticals Inc., Diurnal Limited, Adrenas Therapeutics Inc, Neurocrine Biosciences, Spruce Biosciences, among others.
Congenital Adrenal Hyperplasia Pipeline
Congenital Adrenal Hyperplasia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key congenital adrenal hyperplasia companies, including Neurocrine Biosciences, Spruce Biosciences, Adrenas Therapeutics Inc., Crinetics Pharmaceuticals, Diurnal, among others.
Cushing's Syndrome Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Cushing's syndrome companies including Corcept Therapeutics, Crinetics Pharmaceuticals, Cyclacel Pharmaceuticals, Sparrow Pharmaceuticals, Stero Therapeutics, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
